Table Of Content
- What Is UV Absorber ?
- UV Absorber Classification
- How Does UV absorber Work ?
- Synergistic Effect Of Light Stabilizers And UV Absorber
1. What Is UV Absorber?
These additives preferentially absorb the incident UV radiation and so protect the polymer from the radiation. UV absorbers do not themselves degrade rapidly, but they convert UV energy into harmless levels of heat energy, which are dissipated throughout the polymer matrix. UV absorbers are limited in their effectiveness because of the physical limitations of the absorption process, and their ability to absorb is governed by the need for high concentrations of additive and thickness of polymer before sufficient absorption will occur to retard the photodegradation effectively.
However, high concentrations of additive would be uneconomic and technically limited, while many applications (such as polyolefins) are in very thin sections, such as film and fibre.Benzophenones are good general-purpose UV absorbers for clear polyolefin systems, and can also be used in pigmented compounds. Benzotriazoles are used mainly in polystyrene. Both can also be used in polyesters. Concentrations are usually about 0.25-1.0%
Synonyms of UV absorber
- UV absorber agent
- UV stabilizer
- UV stabilizer agent
- ultraviolet absorber agent
- ultraviolet light absorber agent [dt_divider style="thin" /]
2. UV Absorber Classification
The main function of UV absorbers is to absorb UV radiation in the presence of a chromophore (Ch) found in the polymer, the aim being to filter out the UV light that is harmful to the polymer before Ch* has had a chance of forming. Above all, a UV absorber must function within the 290 and 350 nm range.The purpose of UV absorbers is to absorb harmful UV light and quickly transform it into harmless heat. During this process, absorbed energy is converted into vibrational and rotational energy of the molecule constituents. For UV absorbers to be effective, it is essential that this process take place more rapidly than the corresponding reaction within the substrate, and that neither the UV absorber nor the polymer it is intended to stabilize are damaged during energy conversion. The most important UV absorbers are:
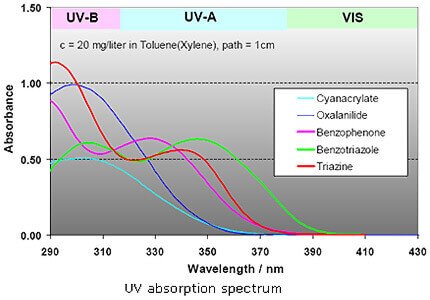
a) 2-(2-hydroxyphenyl)-benzotriazoles
b) 2-hydroxy-benzophenones
c) hydroxyphenyl-s-triazines
d) oxalanilides
Each of these UV absorber groups can be characterized by a typical absorption and transmission spectrum.

3. How Does UV Absorber Work ?
(mechanism of UV absorber)The main function of UV absorbers is to absorb UV radiation in the presence of a chromophore (Ch) found in the polymer, the aim being to filter out the UV light that is harmful to the polymer before chromophore free radical Ch* has had a chance of forming. Above all, a UV absorber must function within the 290 and 350 nm range.The effectiveness of UV absorbers is determined not only by their absorption characteristics but also, above all, by the Lambert-Beer Law.
For a UV absorber to be effective, it must absorb UV light better and faster than the polymer it is meant to stabilize and dissipate the absorbed energy before unwelcome side reactions are triggered.This means that transformation of the energy absorbed in the form of UV light must take place in the singlet state. Inter-system crossing (transition S1 to T1) and therefore phosphorescence must be excluded.
4. Synergistic Effect Of Light Stabilizers And UV Absorber
UV absorbers are not able to absorb all of the UV radiation that a coating is exposed to. Some UV radiation will penetrate the coating surface. Because of this, HALS are incorporated into the polymers. These molecules work by scavenging any free radicals that do form – this is different from UV absorbers, which prevent their formation in the first place HALS function by removing radicals from the system and subsequently regenerating themselves. Most formulators will use a combination of absorbers and HALS for this reason.
Synergistic combinations of UV absorbers and HALS are optimal for the stabilization of polymer. UV absorbers are governed by the Beer Lambert Law, thus absorbance is linearly related to the concentration of UVA (320 to 400 nanometers (used for photocuring), its molar absorptivity (extinction coefficient), and path length (coating thickness). HALS are free radical scavengers and are not subject to Beer's Law and work anywhere in the coating system. HALS are especially effective at coating surfaces, providing better gloss retention, higher chalking resistance in pigmented systems while avoiding crack formation in clear coats. For pigmented systems, HALS provide the primary mechanism of stabilization because most UV radiation is blocked by pigment from penetrating beyond the first few microns of coating. The selection of the appropriate UVA/HALS combinations and concentration is dependent on the chemistry of polymer application system, the presence of pigments and fillers, film thickness and exposure conditions.
